Hydrogen offers the opportunity to store, transport and utilise renewable energy for further applications.
In three minutes the video explains Hartmann’s hydrogen solutions.
Special valves, which have evolved for the demanding hydrogen medium, have been used in the petrochemical industry for many decades. With energy transition, hydrogen will be found increasingly in other fields of application – from electricity generation (such as power-to-gas), through transport (in natural gas grids or hydrogen pipelines), to processing and mobility.
The underground storage of hydrogen in salt caverns constitutes an environmentally friendly and safe solution for storing large amounts of energy to balance between power generation and demand.




Currently around 20 billion m³ of hydrogen is produced in Germany, worldwide it is approximately 500 billion m³. That equates to roughly 2% of the global energy requirement.
The conventional method (around 48%) has for many years been the reforming of natural gas as it is unrivalled in terms of price at manufacturing costs of one Euro per kilogram. This industrial scale generation of hydrogen is carried out in steam reforming plants commonly with capacities of up to 100,000 cubic metres per hour.
Furthermore, hydrogen is also manufactured by partial oxidation of natural gas, liquid hydrocarbons – or in coal-rich countries – also from coal. The hydrocarbons are thereby thermally converted with oxygen into a synthesis gas, a mixture of H2 and CO.
Declining resources of fossil fuels and the battle against climate change have meant that politics and science in the energy sector have for some time now been searching for new ways of generating hydrogen.
Hence processes for the extraction of green hydrogen which is generated exclusively by means of regenerative energy carriers are increasingly coming into focus.
One significant process for the generation of green hydrogen is water electrolysis. This electrochemical process described as power-to-gas utilises wind and sun energy to manufacture hydrogen from electricity and water.
Today there are essentially three electrolysis processes which are used depending on application.
Alkaline electrolysis (AEC): works with caustic potash as the electrolyte at pressures of up to 60 bar at a moderate temperature of 90°C. The anode and cathode chambers are separated by a microporous diaphragm to prevent the mixture of the product gases. This technology is already established and is characterised by its long operating life. With capacities of 0.25 Nm³/h to 1,400 Nm³/h it offers a versatile plant and deployment spectrum but is sluggish in terms of operating behaviour.
Polymer electrolyte membrane electrolysis (PEMEC): works with an ion exchange membrane based on copolymers at pressures of up to 350 bar at a likewise moderate temperature of 90°C. There are already medium-sized plants in existence with capacities of 500 Nm³/h. In contrast to AEC, PEMEC is characterised by a dynamic response behaviour with compact construction but is still very cost intensive in terms of system components.
Solid oxide electrolysis (SOEC): works with solid oxides such as ZrO2 as the electrolyte at temperatures of 700°C to 1000°C. The plants are characterised by a high level of efficiency if the steam required as reactant is available. Until now such plants have only realised up to 40 Nm³/h capacity and due to the high temperature the service life is severely curtailed. The thinking is being directed towards using heat produced in a solar concentrator. Conceivable would be some kind of solar thermal power plant for electricity generation (parabolic trough power plant) in combination with a solar tower power plant in which temperatures of over 1000°C could be generated. This could raise the electrical efficiency of the electrolysis up to 90%. This, however, is only possible in countries with a great deal of direct sunshine.
Besides the electrolysis of water, hydrogen can also be generated by pyrolysis and gasification of biomass. Here, coke and methanol are introduced in the first step of the pyrolysis. In the subsequent reactions with steam and oxygen a gas mixture is formed of hydrogen, CO, CO2 and methane.
Because the resulting CO2 was bonded from the air through the biomass, this method of generation is also considered to be CO2 neutral.
A further approach to CO2-free generation of hydrogen is the Kvaerner process. In this case hydrocarbons such as natural gas or crude oil are split into active carbon and hydrogen at approx. 1600°C by means of a plasma burner. So far, the process has been tested at pilot scale but an industrial scale plant is now planned which should produce 100,000 Nm³ of hydrogen per hour.
The renewable hydrogen produced offers considerable potential for climate protection in an energy system increasingly based on renewable energies. Until now only around 4 % of hydrogen generation is accounted for by this process which is explained by the hitherto even higher manufacturing costs of approx. 6- 10 Euros per kilogram. In the course of the energy transition, however, there is already a multitude of power-to-gas (or power-to-X-) projects and based on this method of hydrogen generation it looks set to capture a strongly increased share of the future energy mix in Germany and even worldwide.
Sources: Sterner /Stadler Hrsg. : Energiespeicher, Bedarf, Technologien, Integration (2. Auflage 2017), DBI 2019, TÜV SÜD 2020
The hydrogen generated can be transported in different ways. Besides transport via a pipeline grid, the hydrogen can also be brought by vehicle from the point of generation to the consumer. Transport in this case is in vessels under high pressure and at ambient temperature or at very low temperature (under -240°C) and low pressure. The major disadvantage of liquid transport at low temperature is the enormous energy requirement for the liquefaction and the energy losses in transit to maintain the low temperature. The losses accrue on the one hand through transfer and on the other through the vaporisation of the liquid hydrogen. The vaporisation process draws energy from the liquid nitrogen which is constantly conducted due to the warmer surroundings and thus maintains the low temperature. Adsorption and metal hydride storage plays a rather more subordinate role as well as the chemical bonding to liquids.
The predominant proportion will thus in the future be transported in pressure vessels at extremely high pressures or in pipeline grids at somewhat lower pressures. With pipeline grids there is the possibility of using special hydrogen lines or admixing the hydrogen to the existing natural gas grid. The addition to natural gas has the disadvantage that the pure hydrogen is no longer directly available but the advantage is that the gas grid is nationwide developed.
For both grids, the hydrogen must be brought up to the line pressure. The compressors, supply lines and valves must be selected for use with hydrogen to guarantee the safety and leak-tightness at all times.
The point of generation and point of use of hydrogen are not only set apart in terms of location but in terms of time. This is exactly as for electricity generation although the hydrogen generated can be significantly better stored. Compared to the electricity grid, which reacts very sensitively to differentiations between production and demand, hydrogen and natural gas transport grids can compensate for fluctuations. The already nationwide developed natural gas grid possesses such a large volume that it can balance differentiations with slight pressure fluctuations. If a greater amount is being supplied than that being drawn off then the pressure rises. It sinks analogously when the withdrawal rate predominates.
In order to balance the hours, days, weeks or even the seasonal variation between summer and winter, however, greater storage is necessary.
Underground salt cavern storage is ideal for this. Where there are large volumes of salt deposits, the salt can be dissolved with water and a cavity thus created. These cavities are leak-tight against hydrocarbons as well as hydrogen and have already been in use for over 50 years for the storage of Germany’s national oil reserve, for example. The seasonal variations in gas requirements are also balanced in Germany using these caverns. The caves are typically established at a depth of 1000 to 1500m and have a diameter of up to 70m and are up to 400m high. The rock pressure there is over 200 bar and the storage pressure can be between 70 bar (emptied) and 200 bar (filled).
Very large amounts of energy can be stored using connected caverns. The capacity of a single cavern is thereby more than 1000 times greater than the world’s largest battery – the “Tesla big battery” in Australia. One reason is its very high volume of up to 1 million geometric cubic metres and the other is that the energy is stored chemically in the H2 molecule form and can be compressed in the caverns. As a result it is far more environmentally friendly in the manufacture of a cavern as no raw materials such as lithium or cobalt are necessary. The storage of hydrogen in caverns for refineries has been used successfully for decades. Examples of this are projects in England and in the south of North America. Ideal conditions for the manufacture of cavern storage can be found in north west Germany. Coincidentally that is also a focal point of the generation of energy from offshore wind farms in the North Sea.
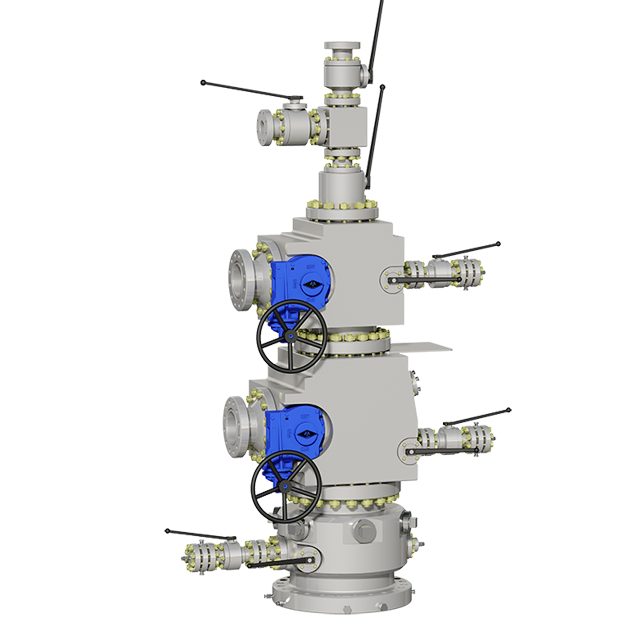
Hartmann wellheads form interface between the above-ground plants and the underground cavern storage. They are adapted to the special characteristics of hydrogen. The first European storage facility in which helium with its likewise very small molecules is stored, and which has been equipped by Hartmann, is also located in Germany.
In addition to the caverns and the wellheads a compressor station also forms part of the plant. This is needed to compress the natural gas or hydrogen from the pipeline pressure of up to approx. 100 bar to 250 bar to pipe it into the caverns. As with generation, plant is required for this pressure increase which is designed for hydrogen. In this regard, apart from the wellheads, Hartmann also supplies the ball valves for the pipelines and the compressor station. Through correct selection of metallic materials and pressure testing with hydrogen the safety and leak-tightness are at all times guaranteed.
Hydrogen has been an important basic material in the most diverse applications for over 100 years. Approximately 19 billion Nm³ of hydrogen are consumed annually in Germany (DWV 2015 (German Hydrogen and Fuel-Cell Association)). Refineries and the chemical industry are responsible for the greatest share of this usage with approx. 85% (DENA 2016 (German Energy Agency)). Of this approx. 30-40% are refinery processes, 25% is used solely for ammoniac manufacture (the worldwide share here is actually approx. 50%) and a further 20% is used in the manufacture of methanol.
Due to changing energy politics towards a decarbonisation of the energy economy, hydrogen, which is being manufactured with low CO2 production processes where possible, is moving ever more into focus as an energy carrier. Apart from industrial use in the chemical, petrochemical and refinery sector, the primary approaches for future usage in this regard are the steel industry, the deployment of H2 through fuel cells for mobility, stationary applications and diverse portable applications.
Even direct reconversion is conceivable but will not foreseeably play any significant role in the energy system in the next ten years. Other representative sectors such as the glass industry, semiconductor industry, plastics production, metal processing and the pharmaceutical industry contribute to less than 1% of hydrogen use (DBI 2020 (German Fuel Institute)).
Ammoniac production (Haber-Bosch process) for the manufacture of fertiliser
Ammoniac is used worldwide as the basis for the production of fertilisers, urea and even explosives. Annual world production is approx. 160 million tons. Ammoniac is manufactured by means of the Haber-Bosch process. Ammoniac synthesis is thereby achieved from atmospheric nitrogen and hydrogen at a ferrous catalyser at pressures of some 150 to 350 bar and temperatures of some 400°C to 530°C, the consumption of hydrogen in the process is between 80,000 m³/h and 160,000 m³/h.
Methanol manufacture
Methanol is the basic material for a multitude of chemical products but in addition its use as a substance is today deployed as an energy carrier. Fuel is produced from methanol with the “methanol-to-gasoline” technology. Methanol is also required in the synthesis of biodiesel and the anti-knocking agent MTBE. By means of fuel cells, electrical energy can be supplied. World annual production is approx. 50 million tons.
Methanol is manufactured in catalytic processes from synthesis gas, a mixture of carbon monoxide and hydrogen in the ration of about 1:2. Depending on the process, operating pressures of 50 to 350 bar and temperatures of 200°C to 380°C are used.
Food chemistry
In the food industry, hydrogen is used in the hardening of vegetable oils. In this process liquid oils are transformed into solid fats (e.g. margarine). Hydrogen is moreover used for the preservation of foodstuffs. In this case the hydrogen, as a packaging gas, inhibits microbial degradation.
Fischer-Tropsch synthesis (manufacture of gasoline, diesel, olefin)
Fischer-Tropsch synthesis is used for the manufacture of liquid hydrocarbons such as gasoline, diesel and olefins. In this industrial scale process for coal liquefaction through the indirect hydrogenation of coal, synthesis gas is initially generated from CO and H2 followed by the transformation into gaseous and liquid hydrocarbons. The process was used in Germany on an industrial scale until 1945 and current plants are to be found in coal rich countries such as South Africa, Malaysia, USA, China, India and Qatar.
Hydro treating processes
1) Hydrodesulphurisation (HDS) / hydro fining
With hydrodesulphurisation (also known as hydro fining / hydro treating), middle distillates (kerosene / gasoil) are desulphurised by hydrogenation of the sulphur compounds. The sulphur is thereby transformed into hydrogen sulphide. The process runs at temperatures between 320°C and 360°C and pressures of 20 to 80 bar. This leads to a considerable reduction in SOx emissions.
2) Hydro metallization (HDM)
In hydro metallization, heavy metals (such as Ni or vanadium) are removed from crude oil using hydrogen.
3) Hydro denitrification (HDN)
With hydro denitrification, organic nitrogen compounds are hydrogenated and transformed into ammoniac. This leads to a considerable reduction in NOx emissions.
4) Hydro deoxygenation (HDO)
With hydro deoxygenation, oxygen is removed from reactants such as light and heavy gasoline, diesel fuel, heating oil and vacuum gasoil through reaction with hydrogen.
The hydrogen requirement in the above stated hydro treatment is 20 to 100 m³/t per ton of input material.
Hydrocracking
In hydrocracking, long chain hydrocarbons are separated out to retain low boiling fractions for diesel and gasoline. The process introduces hydrogen to prevent the formation of solid carbonaceous deposits and increase the yield of saturated hydrocarbon. For process implementation, quantities of up to 500 m³ of hydrogen per ton of input material are necessary.
Hydro formylations
Hydro formylation (also known as oxo synthesis, Roelen reaction) is a technically significant homogenous catalysed reaction in which hydrogen and carbon monoxide are adsorbed on alkenes and other suitable substrates. Products of hydro formylation are aldehydes. The aldehydes are as a rule hydrogenated to alcohols, which are used in a variety of ways as solvents or as intermediate products for manufacturing washing and cleaning agents, lubricants or softeners for plastics.
The manufacture of steel is today one of the most CO2 intensive industry sectors in Germany. Approx. 45 million tons of steel per year are currently produced. To date, steel production for the most part has been based on coal and coke processes for reducing the iron ore in blast furnaces. Huge quantities of CO2 are thereby released into the environment.
There are various approaches to the climate-neutral shaping of steel production through the use of hydrogen. Hitherto, hydrogen has mainly been used in the manufacture of steel as a shield gas / inert gas in the blast furnace.
Substitution of hydrogen for coal /coke as reducing agent in blast furnaces
One approach to decreasing CO2 emissions is the replacement of coal and coke by hydrogen as the reducing agent in the traditional blast furnace process. The first blast furnaces in Germany have been converted in terms of process and others are already being planned. With the conversion of the reducing agent to hydrogen completed, the operators are hoping for a decrease in CO2 of approx. 20% with this process (Thyssenkrupp 2020).
Direct reduction plants
A common alternative to the traditional blast furnace process is offered by the direct reduction of iron ore. Direct reduction plants based on natural gas have been established for many decades and are in use. Here, the iron ore is reduced to sponge iron at temperatures of 1000°C using natural gas instead of coal and coke whereby CO2 emissions are directly diminished. The hydrogen fraction as an admixture to natural gas can thereby be arbitrarily increased and according to a feasibility study, operation is also possible with 100% hydrogen.
As a rule, the hydrogen used has thus far been extracted by means of natural gas steam reforming. In principle the hydrogen requirement in the steel industry can in the future also be covered by green hydrogen. By utilising direct reduction plants with green hydrogen a saving on CO2 emissions of up to 95% is possible in contrast to the traditional blast furnace process (DENA 2018 (German Energy Agency)). Through a complete substitution of the coal /coke requirement an additional hydrogen requirement will accrue in Germany of 2.4 million tons per year (LBST 2017 (Ludwig-Bölkow-Systemtechnik)).
The feature of hydrogen to store renewable energy very efficiently and to be environmentally friendly in deployment far away from electricity generators is one of the most important future applications in the area of mobility. In general there are three possibilities of using hydrogen as propulsion for transport. The direct combustion in normal engines as a substitute for gasoline and diesel, the use of fuel cells and the transformation of green hydrogen into synthetic fuel for use in internal combustion engines.
In the area of transport, hydrogen is mostly deployed by way of fuel cell applications. Fuel cells use the reaction energy resulting from the reaction of hydrogen with atmospheric oxygen in a galvanic cell and convert it into electrical and heat energy.
Because in so doing only water vapour is formed they can be used with versatility as highly efficient and clean electrochemical energy converters. The advantage of H2 fuel cell vehicles is in the high level efficiency of the electrical drive in comparison to combustion engines as well as in the shorter refuelling times and greater range compared to the battery. In just three minutes hydrogen cars can be refuelled with the emission-free fuel at these filling stations for a range of over 500 kilometres – a considerable advantage over pure electric cars. This applies in particular to larger cars, vans and urban local buses and HGVs. The technology in contrast to competing diesel power units is still comparatively expensive as the gas for achieving the equivalent ranges of diesel power units either have to be stored at very high pressures of up to 700 bar or as a liquid at -253°C which is bound with corresponding challenges on the technology. It is, however, already being successfully deployed in all areas of mobility – from cars, HGVs, buses, non-electrified rail traffic, aircraft and rockets to ships and submarines.
In international aviation and shipping, from today’s perspective, due to the high level of demand on the energy density only renewable liquid fuels come into consideration as the alternative to fossil fuels. Hydrogen is thereby a central intermediate product.
Fuels cells work as small thermal power plants. They convert the stored chemical energy not only into electricity but also into heat. The combination also offers itself to decentralised deployment in residential buildings being heated by means of fuel cells which can simultaneously produce electricity. There are two fundamental approaches here for implementation in the area of buildings. One possibility is to furnish households with their own decentralised hydrogen supply from renewable energies.
The second approach is a hydrogen admixture in the existing natural gas grid. The advantage of the hydrogen admixture is that in contrast to the first option the existing infrastructure can be used. An augmented variant in this respect is also the later replacement of natural gas with synthetic methane, manufactured by means of the power-to-X process. The admixture of hydrogen to a maximum tolerance threshold of 20% in the existing natural gas grid is technically, and if needs be, possible and has been researched for some time now (DVGW 2013). H2 admixtures of up to 10% have proven to be unproblematic. In Japan there are already many fuel cells based on natural gas in use and in Germany many prominent heating manufacturers are also already working on such systems.
One highly versatile area for the use of hydrogen in fuel cells is power supply as an alternative both to battery based power supply in the low power range (up to 50W) and engine powered electricity generation in the kW range. The smaller systems are characterised by the high energy content, the larger systems by their environmental friendliness. Thus with this technology not only can small devices like mobile telephones, portable computers, MP3 players and video cameras be operated efficiently but it also has an application for example in grid-independent power supply such as remote locations. With these larger systems the fuel cells as a rule constructed as a cell stack.
Reconversion of green hydrogen can technically take place using the normal natural gas process for power generation, i.e. in gas turbines, combined cycle power plants or with combustion engines. This could have future application in particular during periods of increased power demand and lower renewable energy production.
Gas turbines for use with pure hydrogen below 100MW are already available (Siemens 2019). However, there exists a need for further research to reduce the costs and make the availability of green hydrogen affordable.
A hydrogen admixture in existing gas turbines or natural gas combustion engines is a possibility here to enable the economic operation of such plants in the short term even at lower capacity.
A big challenge so far has been the supply with hydrogen for such plants. In this regard, supply and storage concepts are still to be developed in order to guarantee the delivery and storage of hydrogen in the direct proximity of the power generation plants.
For this reason, the reconversion of hydrogen will not foreseeably be playing any significant role in the energy system for the next 10 years. Due to the huge technical advances of fuel cell engineering in recent years but also through the increased technical requirements on the operation of thermal engines, the energy-orientated use of hydrogen is predominantly seen to be in fuel cell technology.
Sources: DVGW 2013, DWV 2015, DENA 2016, DBI 2019, TÜV SÜD 2020


 Tests
Tests
In future the challenging medium hydrogen will be utilised across increasing fields of application. All components in direct contact with hydrogen must be suitable and tested leak-tight in order to guarantee safe operation. Hydrogen valves and wellheads must therefore meet the design criteria and metallurgy for a leak-tight and secure hydrogen application.
Hartmann offers two hydrogen tests for both Hartmann ball valves and wellheads as well as for the products of other manufacturers (based on documentation).

Molecular hydrogen H2 is comparatively stable and marginally reactive, therefore corrosion in the conventional sense is unlikely. So-called hydrogen embrittlement, i.e. hydrogen induced stress corrosion (see image), presents a risk for highly stressed pressurised components which requires particular consideration. Within the scope of the material suitability test, the material selection is comprehensively tested for hydrogen application.

The test is performed on the basis of the following standards:
The following criteria are considered:

Being a small molecule, hydrogen can migrate through sealing elements. A comprehensive seal test provides assurance that the threshold values are adhered to and fugitive emissions are minimised.

 References
References
Peter Wegjan
p.wegjan[at]hartmann-valves.com
+49 5141 3841-82

Norman Holenstein
n.holenstein[at]hartmann-valves.com
+49 5085 9801-18
Or call us or send us an e-mail. We look forward to your inquiry.
You can reach us by this phone number: +49 5085 9801 0.

The family-owned Hartmann Valves GmbH is one of the leading manufacturers of special ball valves, scraper valves and wellheads. Our high performance components are installed all over the world – in the crude oil, natural gas, (petro)chemical industry as well as in power plant technology, geothermal and other renewables such as power-to-gas.All products are designed to individual requirements and equipped with our metal-to-metal sealing system. Design, assembly and tests are carried out solely in our factories in Celle and Burgdorf-Ehlershausen. Hartmann is now run by the third family generation and have developed into an international system supplier that today employs over 170 people.
A GLIMPSE INTO OUR PRODUCTION:
watch video INDIVIDUAL DESIGN
INDIVIDUAL DESIGNOur sales and design engineers eagerly await your enquiries from the high performance sector. In close collaboration we develop suitable components for your special applications.
 SOLUTIONS FOR EXTREME AREAS
SOLUTIONS FOR EXTREME AREASHartmann ball valves and wellheads are designed for pressure classes up to 690 bar, temperatures from -200 to +550°C as well as for high cycle operations and media of all types.
 QUALITY THAT PAYS
QUALITY THAT PAYSDurable and low maintenance products reduce the life cycle costs of your plants. This is why we develop, assemble and test exclusively in Germany.
 EXPERTISE & CONSULTATION FROM A SINGLE SOURCE
EXPERTISE & CONSULTATION FROM A SINGLE SOURCEYou profit from more than 70 years experience during all project phases. We provide you with support, from consulting, development right through to installation and maintenance service